Synthesis and Applicatin of Iodoethane
Jul 11,2022
Physical data
Iodoethane shows colorless clear heavy liquid, with ether odor. The melting point (°C) is -108 [1]. The boiling point (°C) is 69~73, the relative density (water =1) is 1.95, the relative vapor density (air =1): 5.38, the saturated vapor pressure (kPa) is 13.33 (18.0 °C), the heat of combustion (kJ/mol): -1490.6 [2], the critical pressure (MPa) is 5.99, and the octanol/water partition coefficient is 2.0. Moreover, the flash point (°C) is over 71, the relative density (20°C, 4°C) is 1.9357, normal temperature refractive index (N20) : 1.5133 [3], the normal temperature refractive index (N25) : 1.5101, the liquid phase standard claims heat (enthalpy)(kJ·mol-1) is -39.5, the solubility parameter (J· cm-3) is 19.078, the Van der Waals area (cm2·mol-1) : 5.950×109, and the Van der Waals volume (cm3 ·mol-1) : 43.080. Additionally, the gas phase criterion states heat (enthalpy)(kJ·mol-1) is -7.5, the standard entropy of gas phase (J·mol-1·K-1) is 295.63, the standard free energy of formation in gas phase (kJ·mol-1) is 20.7 [4], and the gas phase standard hot melt (J·mol-1·K-1) is 65.33. Iodoethane is insoluble in water, but soluble in ethanol, ether, hydrocarbon and other organic solvents.
Molecular structure
The molar refractive index is 24.28, the molar volume (CM3 /mol) is 80.3, the constant volume (90.2K) is 185.7, the surface tension (dyne /cm) is 28.5, and the polarization (10-24 cm3) is 9.62.
Preparation
Iodoethane is an important intermediate in organic synthesis, and also an important raw material for the synthesis of MO source [6]. It is used as ethylating reagent, goiter therapy, plant growth stimulant and so on. It is used as a diagnostic aid and analytical reagent in the pharmaceutical industry, as well as in organic synthesis. Now in the production process, the iodide ion residue in the reactor contains 750g/L, which is rich in iodide ion content. Therefore, the recovery of iodide ion from the iodide ion solution into iodide ethane can not only realize the recycling of raw materials of the company, but also realize the harmless treatment of waste liquid. In the existing technology, iodoethane is prepared from red phosphorus, iodine and methanol. This method has large plant investment, low yield, complex separation, and phosphoric acid can not be recovered, resulting in serious envronmental pollution. Therefore, it is very important to recover iodide ions easily to prepare iodide ethane [7]
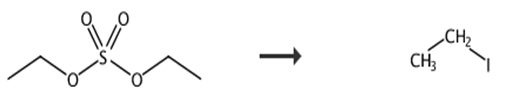
Fig. 1 Synthetic route of iodoethane.
As Fig. 1, the recovered 200 mL iodide ion solution is filtered and analyzed by silver nitrate titration to obtain the iodide ion content of 673g/L. 200ml iodide ion solution is added, stirred, and slowly heated to 70C. Then 180g diethyl sulfate is slowly added to the iodized ion solution for fractionation, while stirring, while receiving the distillate. The reaction kettle temperature is controlled within 90°C, and 152g distillate is received. Then 20g zeolite molecular sieve is added to the collected distillate, stirred for 2h, dried for 24h and filtered to obtain iodoethane. After nuclear magnetic analysis and test, iodoethane content is above 99.9%, the water content is 96ppm and the yield is 91.9% [8].
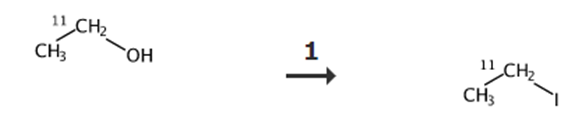
Fig. 2 Synthetic route of iodoethane.
Add ethyl derivative to the concentrated HI (300 μL) at 0 °C.
Heat the reaction mixture to 180 °C.
Distill the reaction mixture through P2O5 and ascarite traps in series into a vial containing 4-nitrophenyl hydrogen methylphosphonate (5 mg, 23 μmol) with Cs2CO3 (9-11 mg, 27-34 μmol) in DMF (300 μL) [9].
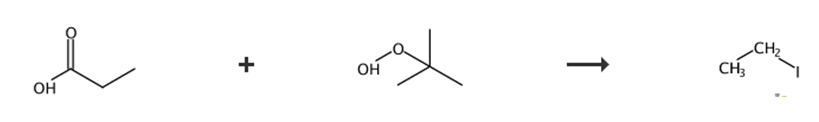
Fig. 3 Synthetic route of iodoethane.
Charge a 100 mL round-bottom flask equipped with a stir bar with CDCl3 (5 mL) and propionic acid (5 mmol).
Cool the mixture to 0 °C.
Add pyridine (5 mmol) dropwise to the vigorously stirred solution.
Stir the solution at 0 °C for 15 minutes.
Add tert-butyl hydroperoxide (3 mmol) and pyridine (5 mmol) dropwise to the stirred solution in sequence.
Stir the solution for 45 minutes.
Warm the solution slowly to room temperature.
Wash the solution with saturated aqueous sodium bicarbonate (3 × 5 mL) and 1 M HCl (2 × 5 mL).
Dry the organic washings over MgSO4.
Concentrate the solution in vacuo (behind a blast shield) to obtain tert-butyl peroxypropionate [10].
Application
Iodoethane is used as a chemical reagent and as a osmotic aid in medicine (to measure cardiac blood output). It is also used in organic synthesis, goiter therapy, plant growth stimulant, etc. In particular, it is widely used as an ethyl reagent in organic synthesis and as a diagnostic aid in the pharmaceutical industry [11].
Stability
Stability: Stability
Forbidden ligand: strong oxidant, strong base
Avoid contact conditions: exposure to heat, light and air
Polymerization harm: no polymerization
Decomposition product: hydrogen iodide
Toxicological information
Acute toxicity: IHL-RAT LC50:65 g/m3/30M
Ipr-mus LD50: 560 mg/kg
Ipr-rat LD50: 330 mg/kg
Orl-rat LD50: 330 mg/kg
Germ cell mutagenicity: MMO-ESC 20 umO /L (-S9)
Dnd-esc: 1 umol/L
LD50:330mg/kg (rat oral): 560mg/kg (mouse oral) [12]
LC50: 65000mg/m3 (rat inhalation, 1/2h)
Mutagenicity of E. coli: 20μmol/L; DNA damage: 1μmol/L.
Storage methods
Iodoethane in 25-500 ml glass bottles shall be protected in wooden cases or cartons. Store in cool, dry, ventilated warehouse, away from fire, heat source, keep away from light. Do not mix with edible chemical raw materials [13].
Storage precautions: Store in a cool and ventilated warehouse. Keep away from fire, heat source and light. Packing should be sealed and out of contact with air. It should be stored separately from oxidizer, alkali and edible chemicals, and must not be mixed. The appropriate variety and quantity of fire fighting equipment is provided. The storage area shall be equipped with leakage emergency treatment equipment and suitable storage material
Reference
[1] K.M. Bulanin, A.G. Shah, A.V. Teplyakov, Infrared spectroscopy studies of iodoethane on Si (100)-2× 1: adsorption and thermal decomposition leading to adsorbate ordering, The Journal of Chemical Physics 115(15) (2001) 7187-7195.
[2] R.J. Clark, J.R. Dann, Matrix isolation study of the photochemically induced reaction between iodoethane and ozone trapped in solid argon at 16 K. Infrared spectra of iodosoethane (C2H5IO), iodylethane (C2H5IO2), ethyl hypoiodide (C2H5OI), hydrogen hypoiodide (HOI), hydrogen iodide, and ethanal complexes, The Journal of Physical Chemistry 100(2) (1996) 532-538.
[3] K. Eapen, Reaction of 2.2-Dichlorotrifluoro-1-iodoethane with zinc, Journal of fluorine chemistry 48(1) (1990) 17-28.
[4] C.E. Dahm, D.G. Peters, Catalytic reduction of iodoethane and 2-iodopropane at carbon electrodes coated with anodically polymerized films of nickel (II) salen, Analytical Chemistry 66(19) (1994) 3117-3123.
[5] Y.-S. Feng, C.-Q. Xie, W.-L. Qiao, H.-J. Xu, Palladium-catalyzed trifluoroethylation of terminal alkynes with 1, 1, 1-trifluoro-2-iodoethane, Organic letters 15(4) (2013) 936-939.
[6] S. Xu, H.-H. Chen, J.-J. Dai, H.-J. Xu, Copper-promoted reductive coupling of aryl iodides with 1, 1, 1-trifluoro-2-iodoethane, Organic letters 16(9) (2014) 2306-2309.
[7] H. Li, J. Sheng, G.X. Liao, B.B. Wu, H.Q. Ni, Y. Li, X.S. Wang, Nickel‐Catalyzed Direct Trifluoroethylation of Aryl Iodides with 1, 1, 1‐Trifluoro‐2‐Iodoethane via Reductive Coupling, Advanced Synthesis & Catalysis 362(23) (2020) 5363-5367.
[8] X. Zheng, D.L. Phillips, A-band resonance Raman spectra and short-time photodissociation dynamics of trans 1-chloro-2-iodoethane in cyclohexane solution, Chemical physics letters 286(1-2) (1998) 79-87.
[9] R. Thissen, M.-J. Hubin-Franskin, M. Furlan, J.-L. Piette, P. Morin, I. Nenner, Selectivity in the fragmentation of 1-bromo-2-iodoethane following iodine 4d core electron excitation, Chemical physics letters 199(1-2) (1992) 102-110.
[10] X. Chen, L. Li, C. Pei, J. Li, D. Zou, Y. Wu, Y. Wu, Visible-Light-Induced Direct Csp2-H Radical Trifluoroethylation of Coumarins with 1, 1, 1-Trifluoro-2-iodoethane (CF3CH2I), The Journal of Organic Chemistry 86(3) (2021) 2772-2783.
[11] A. Nijamudheen, A. Datta, Mechanism for C–I Bond Dissociation in Iodoethane, Iodobenzene, and Iodoethene for the C–C Cross Coupling Reactions over Gold Clusters, The Journal of Physical Chemistry C 117(41) (2013) 21433-21440.
[12] W.M. Kwok, P.K. Ng, G.Z. He, D.L. Phillips, Femtosecond photodissociation dynamics of 1, 1, 1-trifluoro-2The iodoethane in the Franck-Condon region, Molecular Physics 90(1) (1997) 127-139.
[13] Z. Atik, Experimental and predicted volumetric and refractive index properties of ternary mixtures of iodoethane with toluene and alcohols at temperature 298.15 K and pressure 101 kPa, The Journal of Chemical Thermodynamics 38(2) (2006) 201-208.
- Related articles
- Related Qustion
Phenylhydrazine is used in the production of dyes, drugs, developer, etc. It is also an important reagent for identifying carbonyls, aldehydes, ketones, and sugars.....
Jul 11,2022APIFlubenicol sodium succinate is a white or white crystalline powder, odorless and tasteless, easy to deliquescence when the air humidity is high.....
Jul 11,2022APIIodoethane
75-03-6You may like
- ENCH
-

- $100.00 / 1KG
- 2021-07-09
- CAS:75-03-6
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1 Metric Ton/Metric Tons per Month
- Iodoethane
-

- $0.10 / 1KG
- 2020-01-09
- CAS:75-03-6
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 1000 tons
- Iodoethane
-

- $1.00 / 1KG
- 2019-07-14
- CAS:1975-03-6
- Min. Order: 1KG
- Purity: 995
- Supply Ability: 25kg




